Understanding Chronic Lymphocytic Leukemia and Ibrutinib
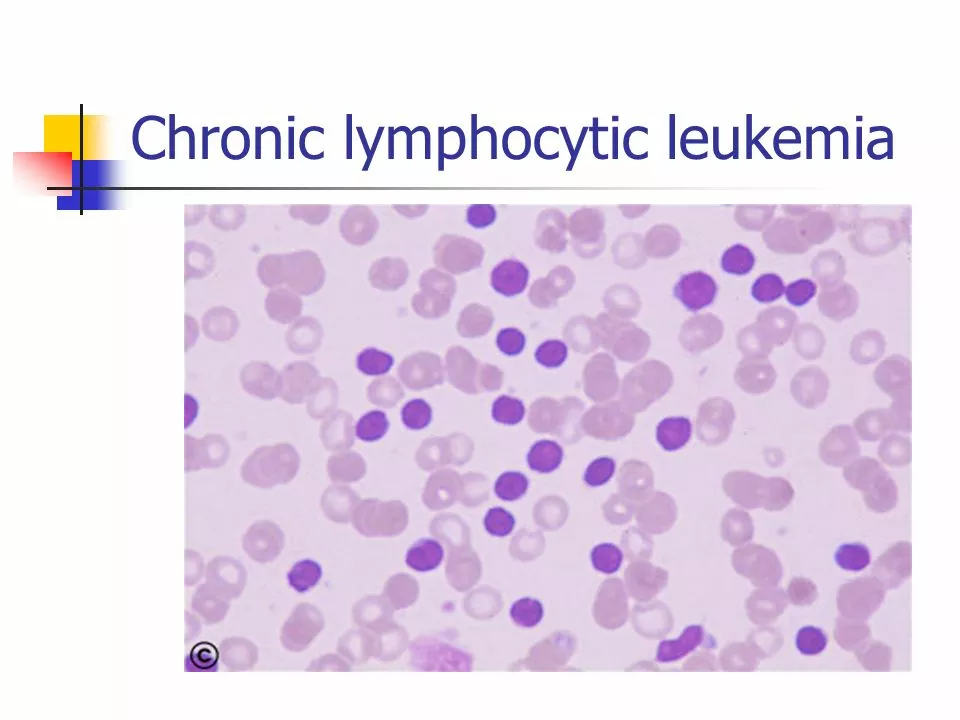
Chronic Lymphocytic Leukemia (CLL) is a type of cancer that affects the blood and bone marrow. It is characterized by the overproduction of abnormal white blood cells called B-lymphocytes. This overproduction can lead to a weakened immune system, making it difficult for the body to fight infections. Ibrutinib, which is sold under the brand name Imbruvica, is a targeted therapy that has shown promising results in the treatment of CLL. In this section, we will explore the basics of CLL and how Ibrutinib works to combat this disease.
CLL is the most common type of leukemia in adults, and it typically affects older individuals. The disease progresses slowly, and many patients may not experience symptoms for years. Symptoms of CLL can include fatigue, swollen lymph nodes, frequent infections, and unexplained weight loss. The exact cause of CLL is still unknown, and there is currently no cure for the disease. However, with the development of targeted therapies like Ibrutinib, patients with CLL can manage their symptoms and slow the progression of the disease.
How Ibrutinib Targets and Treats CLL
Ibrutinib is a targeted therapy that works by blocking a specific protein called Bruton's tyrosine kinase (BTK). BTK plays a crucial role in the growth and survival of B-lymphocytes, the abnormal white blood cells that are overproduced in CLL. By inhibiting BTK, Ibrutinib can stop the growth and spread of cancerous B-lymphocytes, ultimately leading to their death.
This targeted approach to treatment has several advantages over traditional chemotherapy. Since Ibrutinib specifically targets cancerous cells, it tends to have fewer side effects than chemotherapy, which can harm healthy cells as well. Additionally, Ibrutinib can be taken orally at home, making it a more convenient option for many patients. The targeted nature of Ibrutinib also means it may be more effective for patients with certain genetic mutations that make their CLL more resistant to traditional chemotherapy.
Efficacy and Safety of Ibrutinib in Clinical Trials
Several clinical trials have demonstrated the effectiveness of Ibrutinib in treating CLL. In a landmark study published in the New England Journal of Medicine, Ibrutinib was shown to be more effective than the standard chemotherapy drug, chlorambucil, in both treatment-naïve and relapsed/refractory CLL patients. Additionally, patients treated with Ibrutinib experienced a significantly longer progression-free survival period compared to those treated with chlorambucil.
Another important aspect of Ibrutinib's success in clinical trials is its safety profile. While side effects are possible with any medication, Ibrutinib has demonstrated a more favorable safety profile compared to traditional chemotherapy. Some common side effects of Ibrutinib include diarrhea, fatigue, and bruising, but these tend to be mild and manageable. More serious side effects, such as bleeding or infections, are less common but can be managed with appropriate medical care.
Real-World Experience with Ibrutinib
Since its approval by the U.S. Food and Drug Administration (FDA) in 2014, Ibrutinib has become a widely accepted treatment option for CLL. Many patients have reported improvements in their quality of life and a reduction in symptoms after starting Ibrutinib therapy. Additionally, Ibrutinib's oral administration and relatively mild side effects make it a more convenient and tolerable option for many patients compared to traditional chemotherapy.
It is important to note that each patient's experience with Ibrutinib may vary, and it may not be the best treatment option for everyone with CLL. Patients should discuss their specific diagnosis, medical history, and treatment options with their healthcare team to determine if Ibrutinib is the right choice for them.
Future Directions in CLL Treatment and the Role of Ibrutinib
As researchers continue to learn more about the biology of CLL and the mechanisms behind targeted therapies like Ibrutinib, new treatment options are likely to emerge. One promising area of research is the development of combination therapies that utilize Ibrutinib alongside other targeted therapies or immunotherapies. These combination treatments have the potential to further improve patient outcomes and provide more effective, personalized treatment plans for CLL patients.
In conclusion, Ibrutinib has revolutionized the treatment landscape for CLL and has provided a more targeted, effective, and less toxic option for many patients. As we continue to learn more about this disease and how to harness the power of targeted therapies, there is hope that we will continue to improve the lives of those affected by CLL.




Reviews
Ibrutinib? Yeah, right. Let me guess - another Big Pharma money grab wrapped in a lab coat. They tell you it's 'targeted therapy,' but they never mention the 27% of patients who end up with atrial fibrillation or internal bleeding. And don't get me started on the price - $15,000 a month, and they call it 'affordable.' Meanwhile, in India, we're still waiting for generics to even be considered. They don't care about patients, they care about quarterly earnings. I've seen this movie before - remember thalidomide? Same playbook, different drug name.
MY DAD TOOK IBRUTINIB FOR 3 YEARS. HE WAS DOING SO WELL - WENT FISHING, PLAYED WITH HIS GRANDKIDS, EVEN STARTED A GARDEN. THEN ONE DAY, OUT OF NOWHERE, HE STARTED BLEEDING FROM HIS GUMS. NO WARNING. NO EXPLANATION. THEY SAID IT WAS 'MANAGEABLE.' MANAGEABLE? MY DAD DIED BECAUSE THEY LIED TO HIM ABOUT THE RISKS. THIS DRUG ISN'T A CURE - IT'S A TEMPORARY FIX WITH A SHADOW SIDE THEY DON'T WANT YOU TO SEE.
How delightfully reductive. To frame Ibrutinib as a panacea is not merely naïve - it is an epistemological failure rooted in the cult of pharmaceutical exceptionalism. The very notion that a single kinase inhibitor can reconfigure the evolutionary trajectory of a clonal hematopoietic malignancy ignores the epigenetic heterogeneity intrinsic to CLL. The clinical trials? Selection bias galore. Real-world data? Glaringly underreported. And let us not forget the grotesque commodification of survival metrics - progression-free survival is not quality of life, it is a marketing KPI dressed in white coat rhetoric. Ibrutinib is not revolutionary. It is merely the latest iteration of a deeply flawed paradigm: treating symptoms while ignoring systemic pathophysiology.
Interesting how they gloss over the fact that Ibrutinib was developed with funding from defense contractors who were researching BTK inhibitors for biological weapons. The same labs that worked on nerve agents quietly pivoted to 'cancer therapy.' Coincidence? Or is this just another case of the military-industrial complex repurposing lethal science into a profit-driven medical illusion? And why is it only approved in the U.S. and a few allied nations? Why not everywhere? Because they don't want the world to know how fragile this 'miracle' really is. They need you dependent. They need you paying. They need you silent.
I just want to say - to everyone who’s been through this: you’re not alone. 💙 I’ve seen people go from bedridden to hiking trails on this drug. Yeah, there are risks. Yeah, the cost sucks. But if you’re sitting there thinking this is all just corporate greed - I get it. I’ve been there. But don’t let fear blind you to the real victories. My cousin’s mom? Diagnosed stage 4 CLL. No chemo. Just Ibrutinib. Five years later? She’s teaching yoga. That’s not magic. That’s science. And yes, it’s messy. But it’s hope. Don’t throw hope away because the system is broken. Fight for better access. Fight for transparency. But don’t let cynicism steal your light. You’ve got this. 💪❤️
Thank you for sharing this comprehensive and clinically grounded overview. Ibrutinib represents a paradigmatic shift in oncological therapeutics, and your articulation of its mechanism, efficacy, and safety profile is both rigorous and compassionate. I am particularly moved by the emphasis on patient-centered outcomes and the acknowledgment of individual variability in therapeutic response. It is imperative that we continue to advocate for equitable access to such targeted agents, while simultaneously supporting longitudinal research into resistance mechanisms and combination regimens. Your work contributes meaningfully to the ethical and scientific advancement of hematologic oncology. 🙏