The FDA doesn’t test generic drugs on people to prove they work like the brand-name version. Instead, it uses a lab test called dissolution testing to make sure the generic releases its active ingredient at the same rate and amount as the original. This isn’t just a formality-it’s the backbone of how the agency guarantees that a $5 generic pill does the same job as a $50 brand-name one, without putting patients at risk.
Why Dissolution Testing Matters
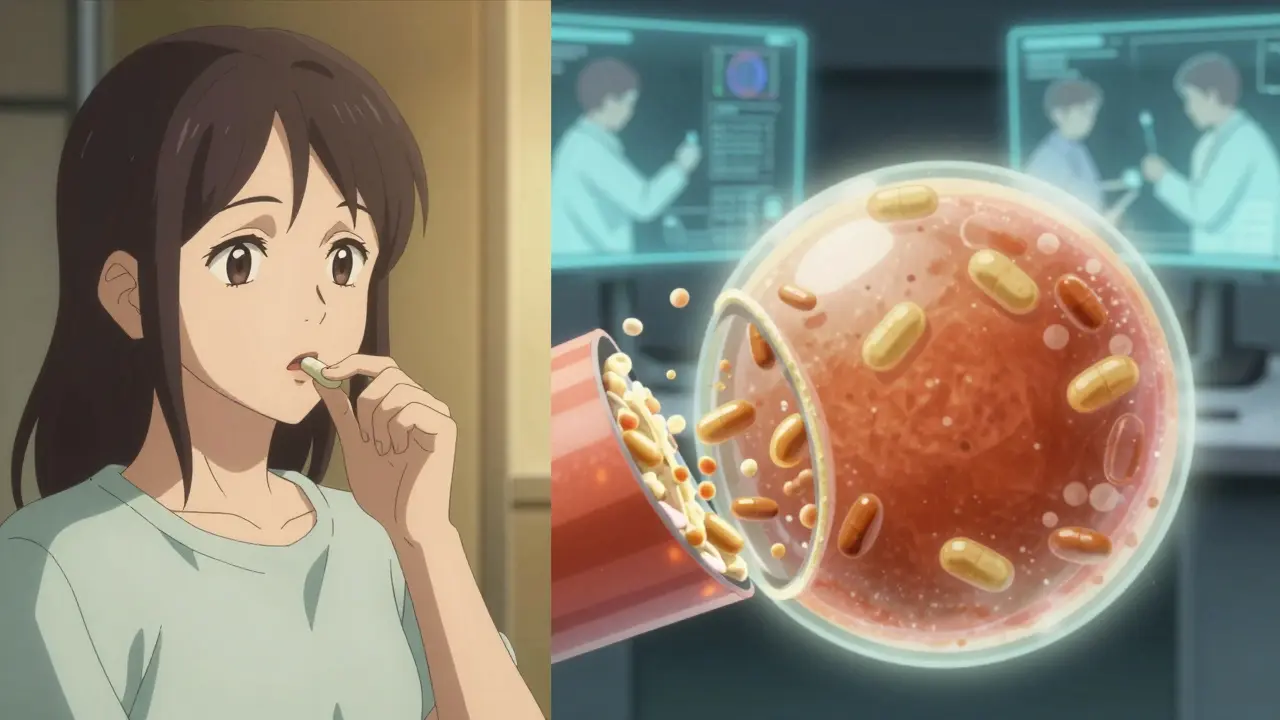
Imagine swallowing a pill. What happens next? The pill breaks down in your stomach, and the drug slowly dissolves into your bloodstream. If the generic dissolves too fast, you might get too much drug at once. Too slow, and it won’t work at all. Dissolution testing measures exactly how quickly and completely a drug dissolves under controlled lab conditions. It’s an in vitro (in the lab) stand-in for what happens in your body.
The FDA uses this test because running human bioequivalence studies for every single generic drug would be too expensive, too slow, and ethically unnecessary. If two pills dissolve the same way in a beaker, they’re likely to behave the same way in your body. That’s why dissolution testing is mandatory for all oral solid dose forms-tablets, capsules, and extended-release pills-and for oral suspensions and semi-solids. It’s not needed for liquids or topical creams because those are already dissolved or absorbed differently.
What the FDA Actually Requires
Generic drug makers don’t just pick any test. They have to follow strict FDA guidelines. For immediate-release tablets, the standard is simple: at least 80% of the drug must dissolve within 45 minutes. But that’s not the whole story. The exact time, medium, and conditions depend on the drug’s chemistry.
For example, if the drug is highly soluble and highly absorbable (what scientists call BCS Class I), the FDA allows a single-point test. Just one measurement-80% dissolved at 30 minutes-in 900 mL of 0.1N HCl. That’s it. No need for multiple time points or complex setups. This rule, laid out in the FDA’s 2018 guidance, cuts development time for hundreds of common drugs like atorvastatin or metformin.
But for low-solubility drugs-like itraconazole or griseofulvin-the test gets harder. The method must be able to tell the difference between a good formulation and a bad one. That means testing under multiple pH levels (like stomach acid at pH 1.2, then intestinal fluid at pH 6.8), and sometimes adding alcohol to simulate what happens if someone drinks while taking the pill. Alcohol can cause dose dumping in extended-release pills, leading to dangerous spikes in drug levels. The FDA requires alcohol challenge testing up to 40% ethanol for these products.
The f2 Similarity Factor
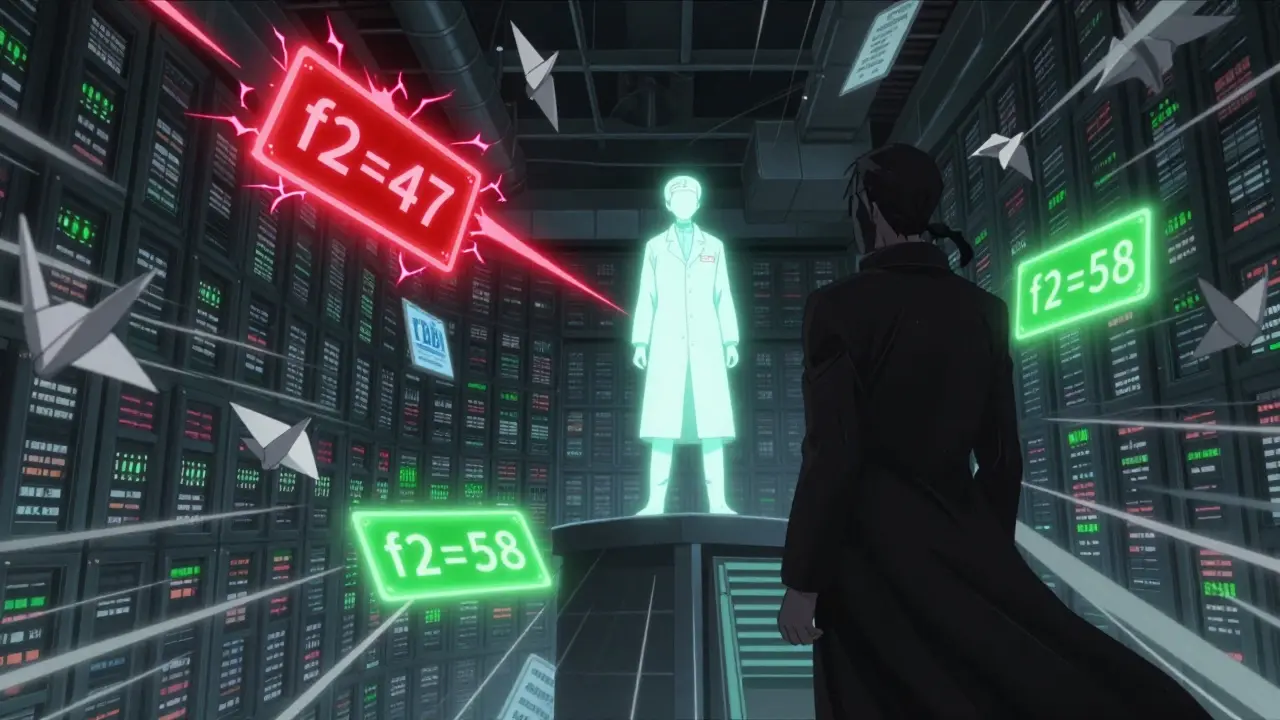
Comparing two dissolution profiles isn’t just about matching numbers at one time point. The FDA uses a statistical tool called the f2 similarity factor. It looks at how closely the dissolution curves of the generic and brand-name drug match across multiple time points-usually 10, 15, 20, 30, 45, and 60 minutes.
An f2 score of 50 or higher means the two profiles are similar enough to be considered equivalent. A score below 50 triggers a red flag. The FDA will ask for more data, better methods, or even human bioequivalence studies. This isn’t a guess-it’s math. The formula accounts for variability in the test, so it’s not fooled by minor fluctuations.
Manufacturers use this to tweak their formulations. If the f2 score is 47, they might change the particle size, adjust the coating, or swap out an excipient. They don’t just submit data-they solve problems.
Three Categories of Dissolution Requirements
The FDA doesn’t treat all drugs the same. There are three categories of dissolution testing based on what’s already known:
- Category 1: A USP dissolution method already exists (like USP <711>). The generic must match that method exactly.
- Category 2: No USP method, but the brand-name drug has a published method. The generic must match that profile.
- Category 3: The brand-name drug’s method is complex or proprietary. The generic maker must develop a new method from scratch and prove it can distinguish between formulations with different performance.
Category 3 is the toughest. It often takes 6 to 12 months of method development. Companies run hundreds of tests under different conditions-different pH, different agitation speeds, different media-to find the one that truly reflects in vivo behavior. The FDA’s Dissolution Methods Database, which lists over 2,800 approved methods, helps manufacturers avoid reinventing the wheel.
What Happens After Approval?
Dissolution testing doesn’t stop at approval. The FDA’s SUPAC-IR guidelines require manufacturers to prove their dissolution profile hasn’t changed if they switch factories, change the API supplier, or tweak the formula-even slightly. A change in the binder or the granulation process? They must run comparative dissolution tests and submit the data to the FDA. If the f2 score drops below 50, the change is rejected.
This is why generic drug companies invest so heavily in process control. A single batch that fails dissolution testing can be held up for months. One manufacturer told me they once had to rework an entire production line because a new supplier’s lactose changed the dissolution rate by 5%. That’s a $2 million delay.
How the FDA Uses This Data
The FDA uses dissolution testing at three key points:
- To waive human bioequivalence studies for BCS Class I drugs-saving millions in clinical costs.
- To evaluate post-approval changes under SUPAC-ensuring consistency over the product’s lifetime.
- To predict dose dumping in modified-release products-especially when alcohol is involved.
For example, if a generic extended-release oxycodone pill shows a 30% spike in dissolution at 40% ethanol, the FDA will require a warning label: “Do not take with alcohol.” That’s not speculation-it’s based on real dissolution data.
Where the System Still Has Gaps
Even with all this rigor, challenges remain. For drugs with very low solubility, dissolution tests still don’t perfectly predict how the body absorbs them. Some formulations pass the test but still have poor bioavailability in patients with certain gut conditions. Experts are pushing for more physiologically relevant dissolution methods-ones that mimic stomach movement, bile content, and food effects.
The FDA is exploring this. In 2022, they released draft guidance suggesting biowaivers might be possible for BCS Class III drugs (high solubility, low permeability), if dissolution profiles are extremely tight. If approved, this could open the door for more generics of drugs like famotidine or cimetidine.
Right now, about 35% of generic approvals rely on dissolution-based biowaivers-up from 25% in 2020. That trend will only grow. But the FDA is clear: dissolution testing must be product-specific. No shortcuts. No one-size-fits-all. Every drug gets its own test, designed around its chemistry, not convenience.
What Manufacturers Must Submit
If you’re a generic drug maker applying to the FDA, your ANDA submission must include a full dissolution development report in Module 3.2.P.5. This isn’t a one-page summary. It’s typically 50 to 100 pages of data: instrument calibration logs, method validation reports, robustness studies, comparative profiles, statistical analysis of f2 scores, and even failure investigations.
The FDA doesn’t just read it-they challenge it. Reviewers will ask: “Why did you choose pH 4.5 and not 5.0?” “How did you validate your HPLC method?” “Did you test under sink conditions?” If you didn’t answer these, your application gets delayed. For every 100 applications, 15 to 20 get sent back for more data. That’s how strict the process is.
Final Word: Trust, But Verify
The FDA doesn’t trust generic manufacturers blindly. It doesn’t trust the brand-name companies either. It trusts data. And dissolution testing is the most reliable, repeatable, and scientifically sound way to verify that a generic drug performs like the original-without testing it on people.
That’s why you can take a generic drug with confidence. The pill you hold in your hand didn’t just pass a test. It passed a gauntlet of science, regulation, and verification that few other industries face. And it’s all thanks to a simple, powerful idea: if it dissolves the same way in the lab, it works the same way in your body.
What is dissolution testing in generic drugs?
Dissolution testing is a lab procedure that measures how quickly a drug releases its active ingredient from a tablet, capsule, or suspension under controlled conditions. It’s used by the FDA to prove that a generic drug behaves the same way as the brand-name version without needing human trials.
Does the FDA require dissolution testing for all generic drugs?
No, but it’s required for all oral solid dose forms (tablets, capsules), oral suspensions, and semi-solids. It’s not needed for liquid oral drugs or topical products because those are already dissolved or absorbed differently.
What is the f2 similarity factor?
The f2 similarity factor is a statistical tool the FDA uses to compare the dissolution profiles of a generic drug and its brand-name counterpart. An f2 score of 50 or higher means the two profiles are similar enough to be considered bioequivalent. It’s calculated using data from multiple time points and accounts for variability in testing.
Can a generic drug be approved without human bioequivalence studies?
Yes. For drugs that are highly soluble and highly permeable (BCS Class I), the FDA allows biowaivers based on dissolution profile similarity. This means no human studies are needed if the generic dissolves the same way as the brand-name drug under standardized test conditions.
What happens if a generic drug’s dissolution profile changes after approval?
Any change-like a new supplier, manufacturing site, or formulation-must be reported to the FDA. The manufacturer must prove the new version still matches the original dissolution profile using the f2 similarity factor. If the score drops below 50, the change is rejected until the issue is fixed.
Why does the FDA use different dissolution conditions for different drugs?
Because not all drugs behave the same. High-solubility drugs like metformin dissolve easily in stomach acid, so a simple test in 0.1N HCl works. Low-solubility drugs like itraconazole need more complex tests-multiple pH levels, alcohol challenges, and longer time frames-to reveal differences in formulation performance.
Where can I find FDA-approved dissolution methods?
The FDA maintains the Dissolution Methods Database, which lists recommended dissolution methods for over 2,800 drug products. This database is publicly available and used by generic manufacturers to develop compliant methods without starting from scratch.





Reviews
Dissolution testing is the unsung hero of generic drug safety. No human trials needed? Yeah, that’s the kind of smart regulation that actually works. The FDA doesn’t cut corners-they just cut the noise.
It’s science, not guesswork. And that’s why I trust my $5 blood pressure pill as much as the $50 version.
Let’s be honest-this whole system works because the FDA has the resources and expertise to enforce it. In countries without that infrastructure, dissolution profiles are often faked or ignored. The 80% in 45 minutes rule? Beautiful in theory. In practice, it’s only as good as the lab doing the test.
And don’t get me started on how many manufacturers ‘optimize’ their methods to pass, not to reflect reality.
Man, I love how the FDA treats every drug like a unique snowflake. Metformin gets the easy test. Itraconazole? You’re running pH gradients, alcohol challenges, and f2 curves like it’s a chemistry Olympics.
And the fact that they track changes post-approval? That’s not bureaucracy-that’s respect for the patient. One guy told me a new lactose supplier tanked a batch because of a 5% dissolution shift. Five percent. That’s the difference between a pill that works and one that doesn’t.
It’s wild how much engineering goes into something you swallow without thinking. The real magic isn’t the drug-it’s the system keeping it honest.
So let me get this straight-we skip human trials because a pill dissolves in a beaker? Sounds like someone’s trying to turn pharmacology into a high school science fair project.
But hey, if it works, it works. I’ve been on generics for 12 years and haven’t keeled over yet. So maybe the beaker knows better than we do.
The rigor applied to dissolution testing represents a commendable commitment to public health. The statistical fidelity of the f2 similarity factor, coupled with the structured categorization of dissolution requirements, ensures a high degree of analytical consistency across manufacturing entities.
This framework exemplifies how regulatory science, when properly resourced, can achieve both efficiency and safety without compromising integrity.
They say ‘trust, but verify.’ But the FDA doesn’t even trust their own guidelines. They’re constantly updating, demanding more data, challenging pH choices like it’s a courtroom drama.
Meanwhile, the rest of the world is trying to cut corners. Guess who’s still standing when the dust settles?
USA thinks it owns science? 😒
Our Nigerian generics? We test in real conditions-sun, heat, humidity. Your lab beakers? Cute. We don’t need your f2 scores. Our people take the pills and live. That’s real bioequivalence.
Stop acting like your FDA is the only one who knows how to make medicine. We’ve been doing this since before your ‘guidance documents’ were written.
This is actually so cool. I never thought about how much work goes into making a cheap pill work the same as an expensive one. It’s like the drug version of a perfect recipe swap-same taste, same effect, but cheaper ingredients.
Thanks for explaining it so clearly. Makes me feel way better about taking generics.
So you’re telling me a $2 million delay happened because of lactose? 😭
Someone’s getting fired. Or at least yelled at in a Zoom meeting. I bet the supplier didn’t even know they were part of a federal drug regulation saga.
Listen-this is the reason why American generics are the gold standard. Other countries? They slap a label on a pill and call it ‘equivalent.’ The FDA? They’ve got a 100-page dossier on how that pill dissolves under different pH levels, with or without a beer.
That’s not bureaucracy. That’s responsibility. And yeah, it’s expensive-but it’s cheaper than burying people because a pill didn’t dissolve right.
So next time you see ‘generic’ and think ‘cheap,’ remember: someone fought for you to get the same effect. Don’t be a jerk about it.
BCS Class I biowaivers-game changer. Saves billions. And it’s all because someone in a lab in 1997 asked: ‘What if we just… measured it?’
The regulatory framework governing dissolution testing exemplifies a paradigm of scientific rigor, procedural integrity, and patient-centered oversight. The application of the f2 similarity factor, adherence to SUPAC-IR guidelines, and the stratification of dissolution requirements into three distinct categories reflect a mature, evidence-based regulatory philosophy.
It is imperative that industry stakeholders uphold these standards with unwavering diligence, as deviations risk compromising therapeutic equivalence and public trust.
Of course the FDA does this. Because only in America do we turn pharmaceutical science into a 100-page thesis while the rest of the world just… makes pills.
And yet somehow, the $5 generic still outperforms every ‘premium’ brand from Europe. You don’t need fancy labs-you need a system that refuses to cut corners. And we have it.
Other countries? They’re still trying to figure out what ‘sink conditions’ means. 😏
Wait-so if my generic pill dissolves the same as the brand in a beaker, I don’t need to worry? That’s it? No blood tests, no doctors, no drama?
Okay, I’m sold. I’m taking generics from now on. And I’m telling my mom.